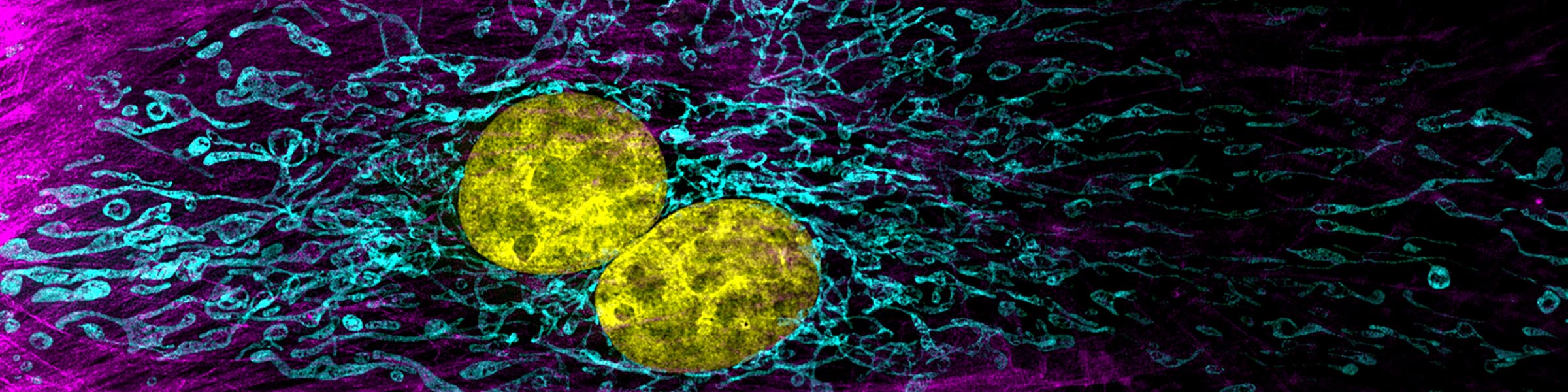
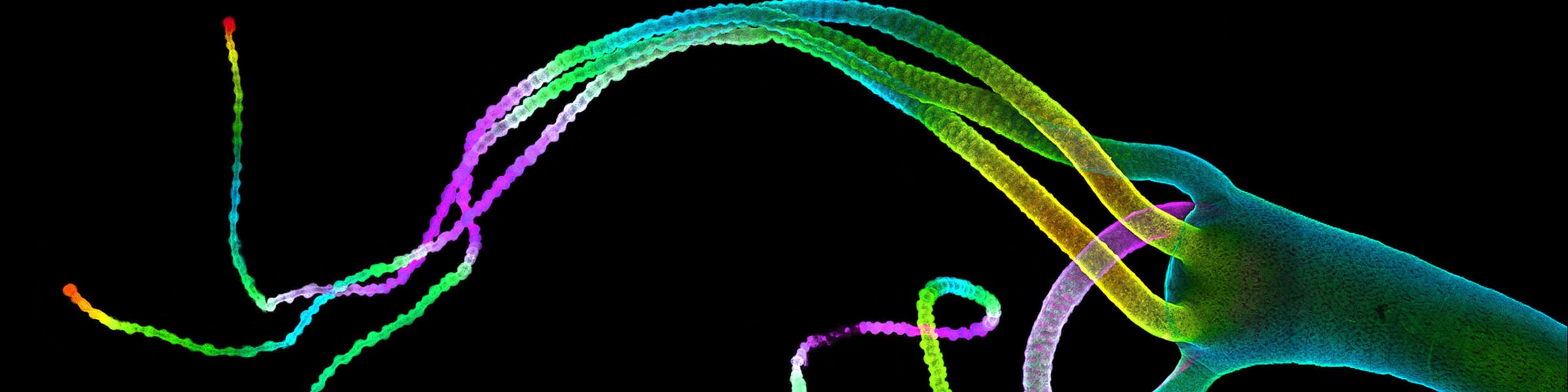
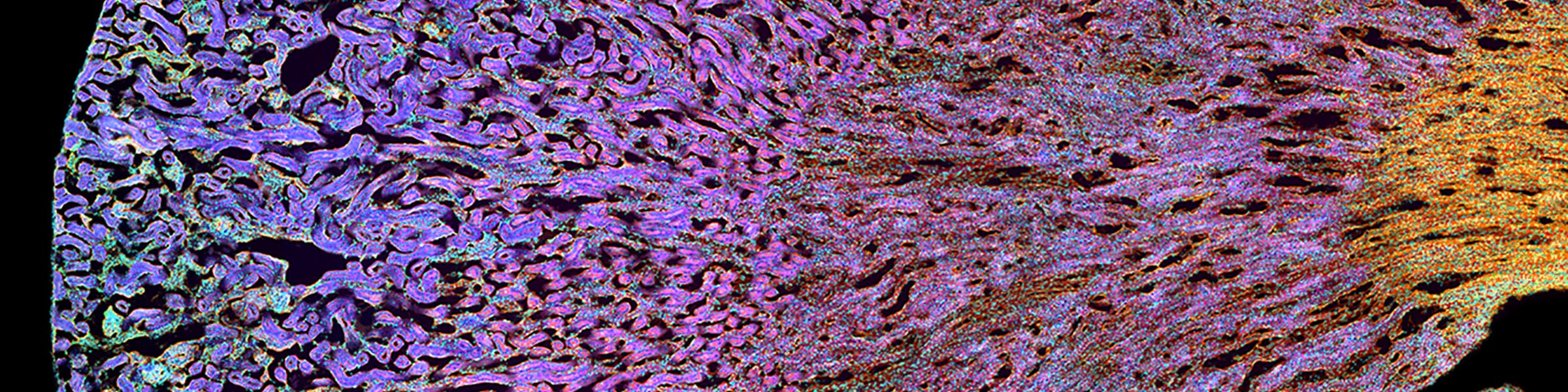
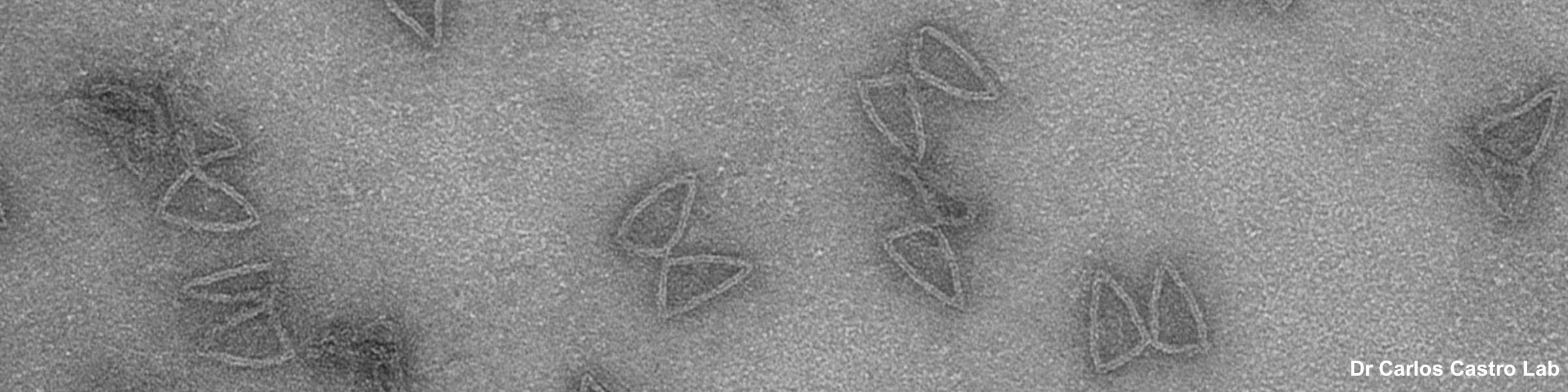
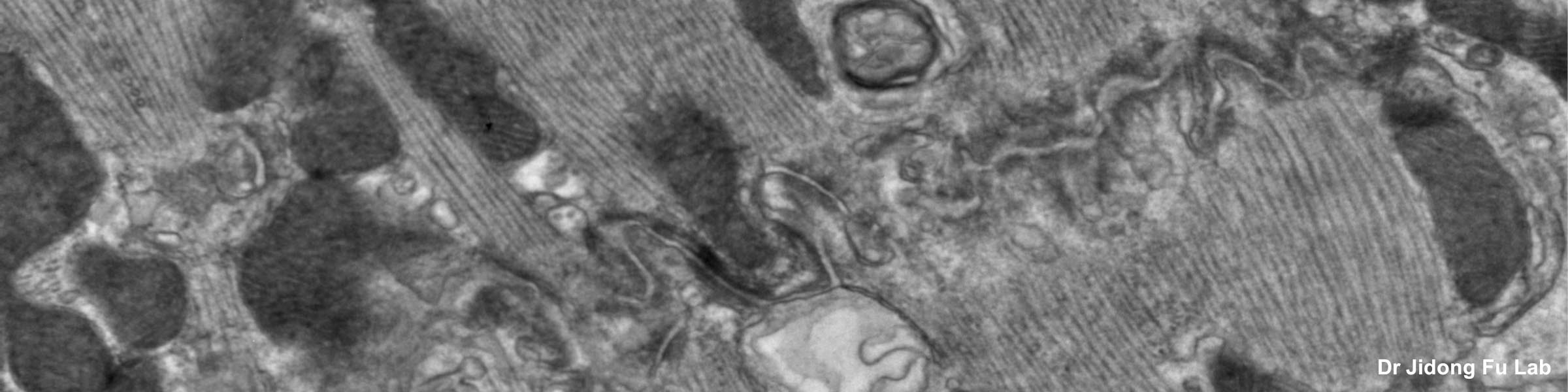
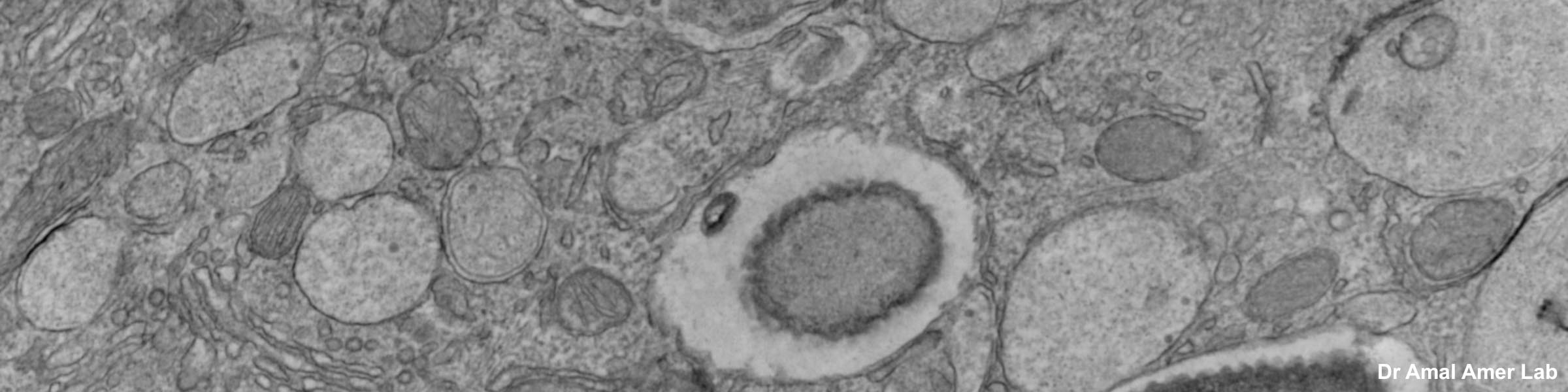
Fixation and dehydration for the SEM are carried out similarly to those for the TEM (see TEM fixation protocol). There are many variations based on the type of tissue to be examined, whether one is dealing with cell suspensions, biopsies, perfused tissues, or monolayer cultures. Because most SEM involves examination of surface structures, fixation-penetration is generally less critical than for TEM and much larger samples may be processed. The times in primary fixative, buffers, and alcohol may be extended if the tissue pieces are large. After dehydration through an ascending series of ethanols ending in 100% ethanol, the SEM protocol requires drying without introducing surface tension artifacts. This can be done by using our Pelco critical point drier or by drying from the chemical HMDS, which is available from Ted Pella, Inc., as well as from many other electron microscopy suppliers.
After drying, the samples are carefully mounted on an aluminum stub using either silver paint or a double stick carbon tape. Samples are then introduced into the chamber of the sputter coater and coated with a very thin film of gold\palladium before SEM examination.
In all cases, a discussion with the staff at CMIF prior to beginning the protocol is strongly suggested.
Basic SEM Protocol for cells grown on glass coverslips
After drying, the samples are carefully mounted on an aluminum stub using either silver paint or a double stick carbon tape. Samples are then introduced into the chamber of the sputter coater and coated with a very thin film of gold\palladium before SEM examination.
In all cases, a discussion with the staff at CMIF prior to beginning the protocol is strongly suggested.
Basic SEM Protocol for cells grown on glass coverslips
- Completely rinse any growth media off the cells. ( Usually PBS is appropriate)
- Fix at room temperature for 2 hours with SEM Fixative (Provided by The CMIF)
- Rinse 3 times using the same buffer used for the fixative for 5 minutes each rinse
- Post-fix for 1 hour in 1% osmium tetroxide in the same buffer
- Rinse 3 times using the same buffer for 5 minutes each rinse (can hold sample up to 72 hours at 4C at this point prior to dehydration)
- 50% ethanol – 10 minutes
- 70% ethanol – 10 minutes
- 80% ethanol – 10 minutes
- 95% ethanol – With 2 changes within 10 minutes
- 100% ethanol – from a newly opened bottle - 3 changes within 15 minutes (can hold sample up to 3 hours at RT at this point)
- Critical-point dry or chemically dry using HMDS