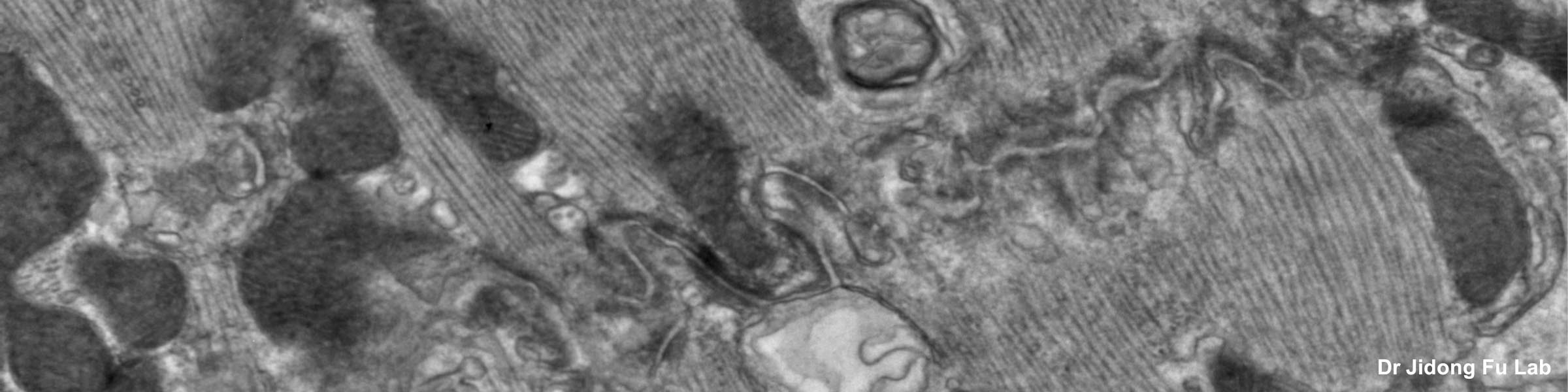
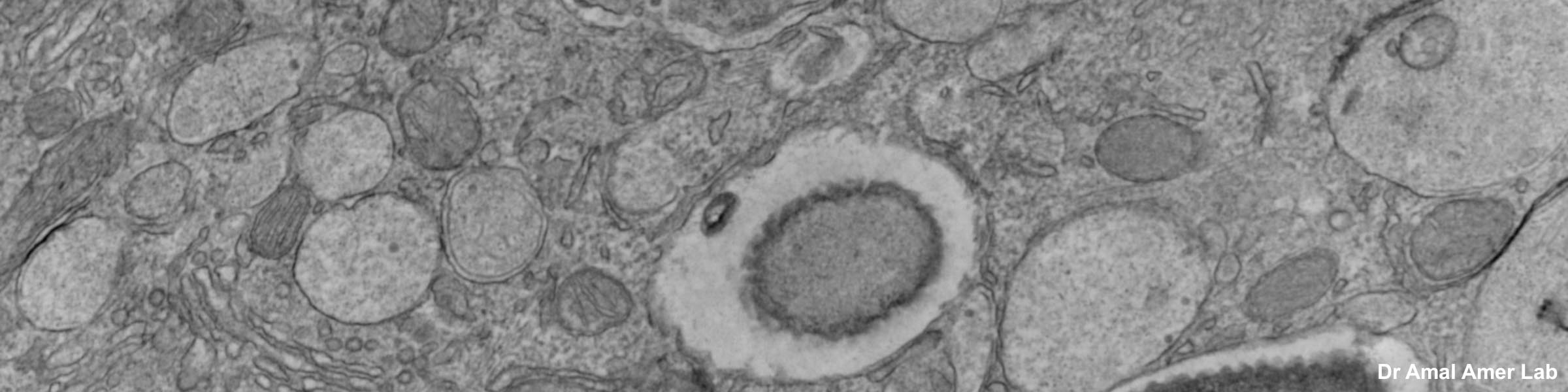
Before a piece of tissue can be examined in the transmission electron microscope, it must be properly fixed, dehydrated, infiltrated with resin, embedded, polymerized, sectioned, and stained. This is a very basic description. There are many variations based on the type of tissue to be examined, whether one is dealing with cell suspensions, biopsies, perfused tissues, or monolayer cultures. A highly recommended reading for those with limited background in biological electron microscopy is the book: Electron Microscopy, Principles and Techniques for Biologists, by John J. Bozzola and Lonnie D. Russell, published by Jones and Bartlett, 1992. ISBN number is 0-86720-126-6. In all cases, a discussion with the staff at CMIF prior to beginning the protocol is strongly suggested.
- Excise the piece of tissue and immediately put it into a puddle of fixative on a wax plate. Cut the tissue into thin slices and allow them to harden in the fixative for about 10 minutes before further cutting. Ultimately the pieces should not exceed 1 mm cubed.
- Fix the tissue for 1-2 hours at room temperature.
- Rinse the tissue 3 times in an appropriate buffer for 5 minutes each rinse.
- Post-fix the tissue for 1 hour in 1% osmium tetroxide in an appropriate buffer or in distilled water.
- Rinse 3 times in buffer (or distilled water if used with osmium) for 5 minutes each rinse.
- 50% ethanol - 10 minutes
- 70% ethanol - 10 minutes
- 80% ethanol - 10 minutes
- 95% ethanol with 2 changes within 10 minutes
- 100% ethanol from a newly opened bottle - 3 changes within 15 minutes.
- Propylene oxide - 10 minutes
- 1:1 propylene oxide\resin - minimum of 1 hour
- 1:2 propylene oxide\resin - 1 hour or overnight on a rotator.
- 100% Epon or Spurr resin - 2 changes over 2-6 hours.