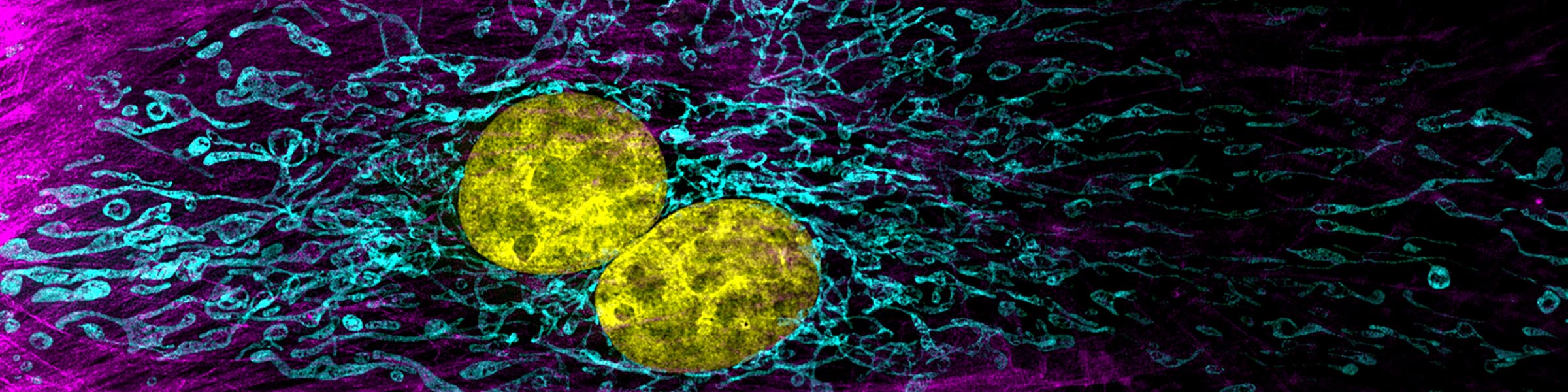
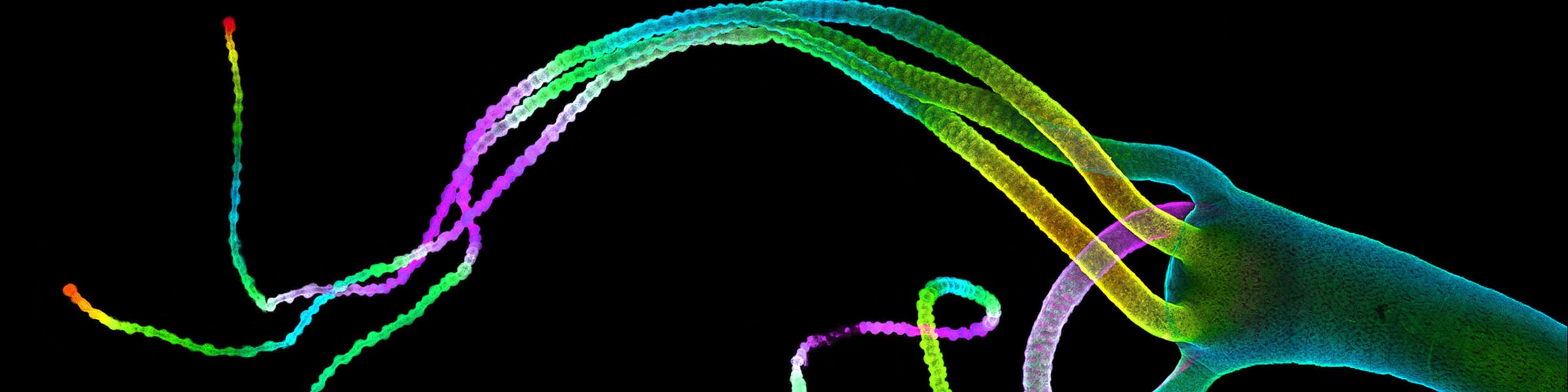
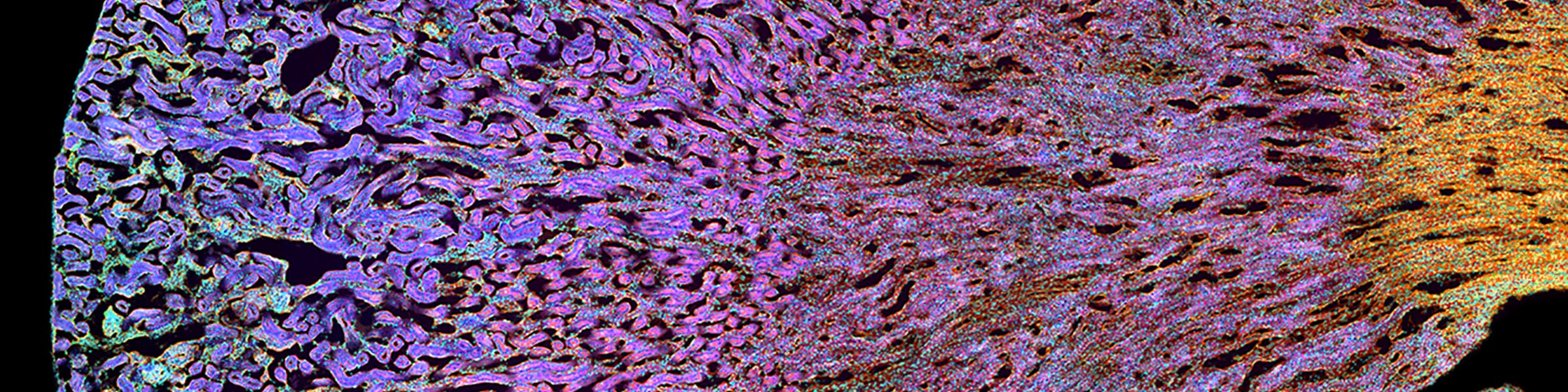
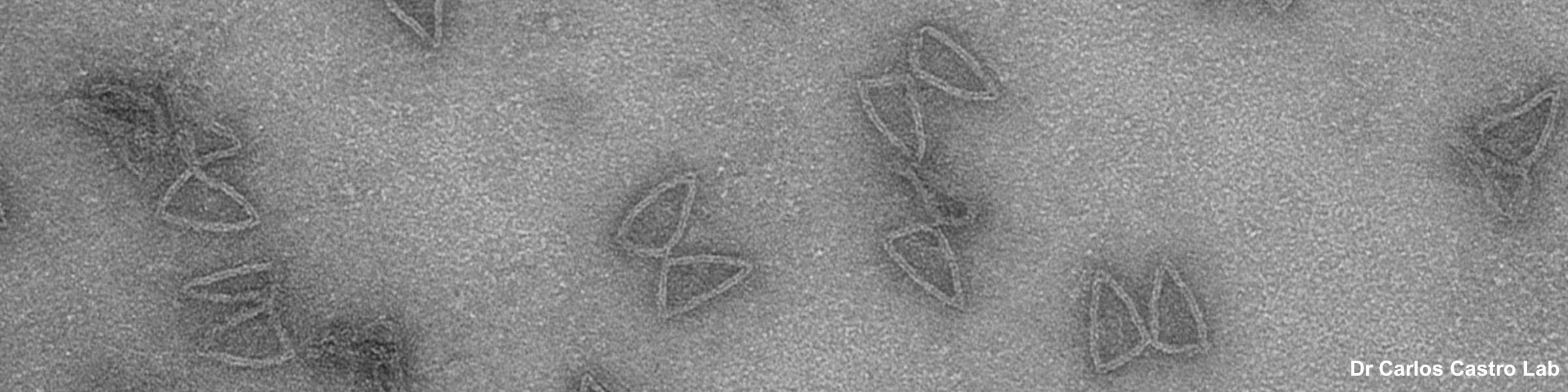
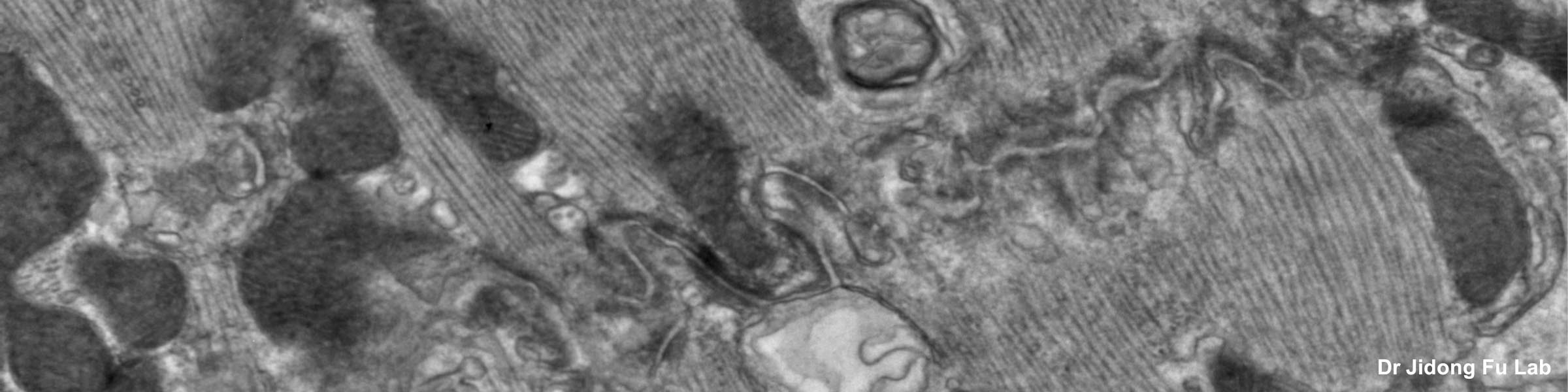
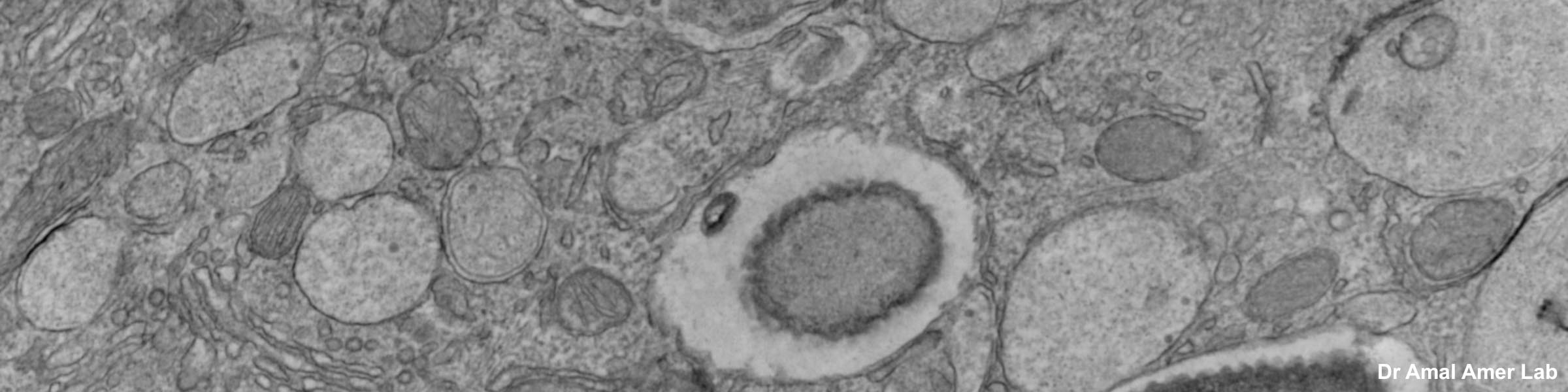
Rinse cells well with Phosphate buffer to remove residual media and serum (critical for SEM applications). Fix cells in 4% paraformaldehyde at room temp. Remove fix and wash 2x with Phosphate Buffer.
Fluorescence-based Immunocytochemistry:
Permeabilization:
1. Incubate the samples for 10 min with buffer containing 0.25% Triton X-100 (or alternative detergent) at room temperature.
2. Wash three times for 5 min each using PBS.
Blocking and Incubation:
3. Incubate cells with 1% BSA in PBS for 30 min to block non-specific binding of the antibodies. Filter BSA using a 0.2 micron syringe filter. Alternative blocking solution - 10% serum from the species that the secondary antibody was raised in.
4. Dilute primary antibody in 1% BSA in PBS and apply to sample. Incubate in a humidified chamber for 1 hr at room temperature or overnight at 4°C. Protect samples from light.
5. Remove primary antibody and wash the cells three times in PBS, 5 min each wash.
6. Incubate cells with the secondary antibody (diluted in 1% BSA) for 1 hr at room temperature protected from light.
7. Remove the secondary antibody solution and wash 5-7 times with PBS for 5 min.
Counter staining (optional):
8. Incubate cells on 0.1-1 μg/ml Hoechst or DAPI (DNA stain) for 1 min. (Can be included in one of the final washes)
9. Rinse briefly with dH2O to remove residual salt.
Mounting coverslip:
10. Mount #1.5 coverslip with a drop of aqueous mounting medium. Use the coverslip appropriate for the microscope optics that will be used for analysis.
Fluorescence-based Immunocytochemistry:
Permeabilization:
1. Incubate the samples for 10 min with buffer containing 0.25% Triton X-100 (or alternative detergent) at room temperature.
2. Wash three times for 5 min each using PBS.
Blocking and Incubation:
3. Incubate cells with 1% BSA in PBS for 30 min to block non-specific binding of the antibodies. Filter BSA using a 0.2 micron syringe filter. Alternative blocking solution - 10% serum from the species that the secondary antibody was raised in.
4. Dilute primary antibody in 1% BSA in PBS and apply to sample. Incubate in a humidified chamber for 1 hr at room temperature or overnight at 4°C. Protect samples from light.
5. Remove primary antibody and wash the cells three times in PBS, 5 min each wash.
6. Incubate cells with the secondary antibody (diluted in 1% BSA) for 1 hr at room temperature protected from light.
7. Remove the secondary antibody solution and wash 5-7 times with PBS for 5 min.
Counter staining (optional):
8. Incubate cells on 0.1-1 μg/ml Hoechst or DAPI (DNA stain) for 1 min. (Can be included in one of the final washes)
9. Rinse briefly with dH2O to remove residual salt.
Mounting coverslip:
10. Mount #1.5 coverslip with a drop of aqueous mounting medium. Use the coverslip appropriate for the microscope optics that will be used for analysis.