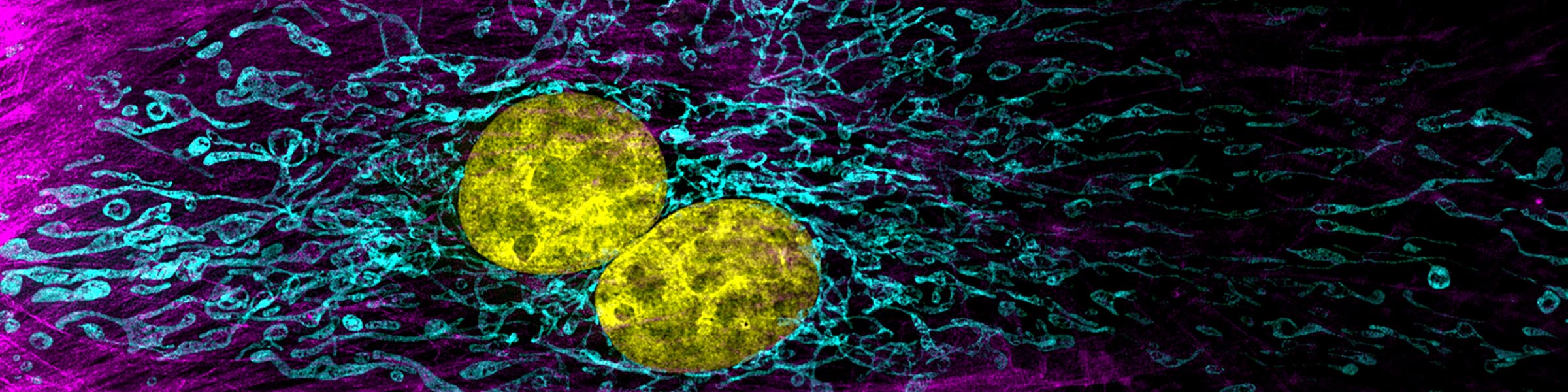
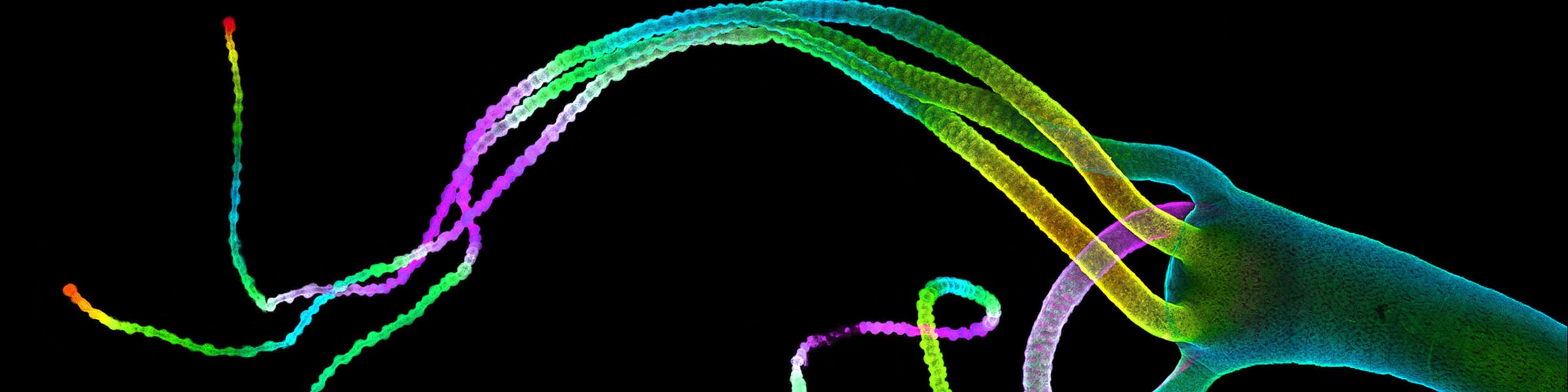
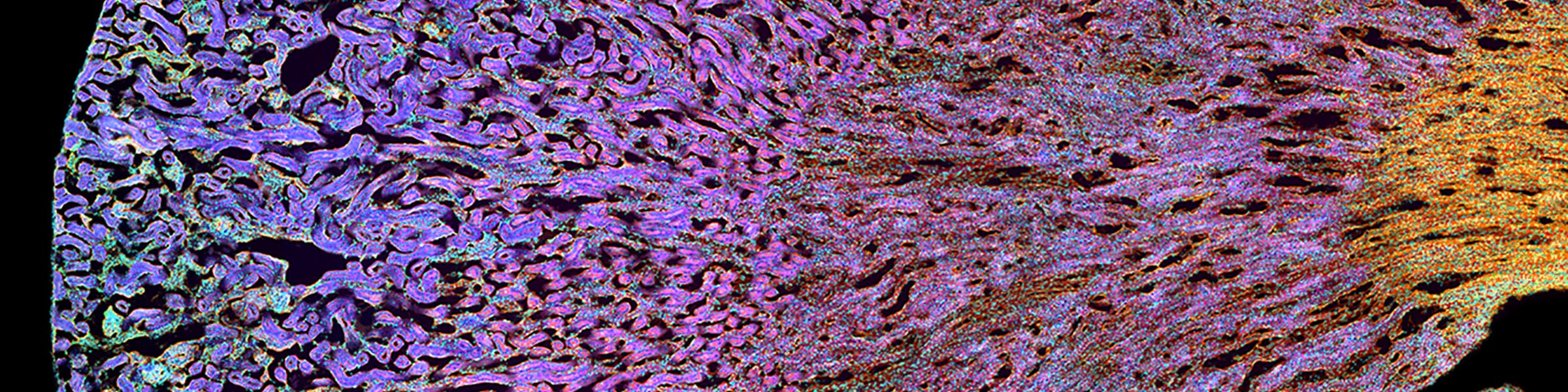
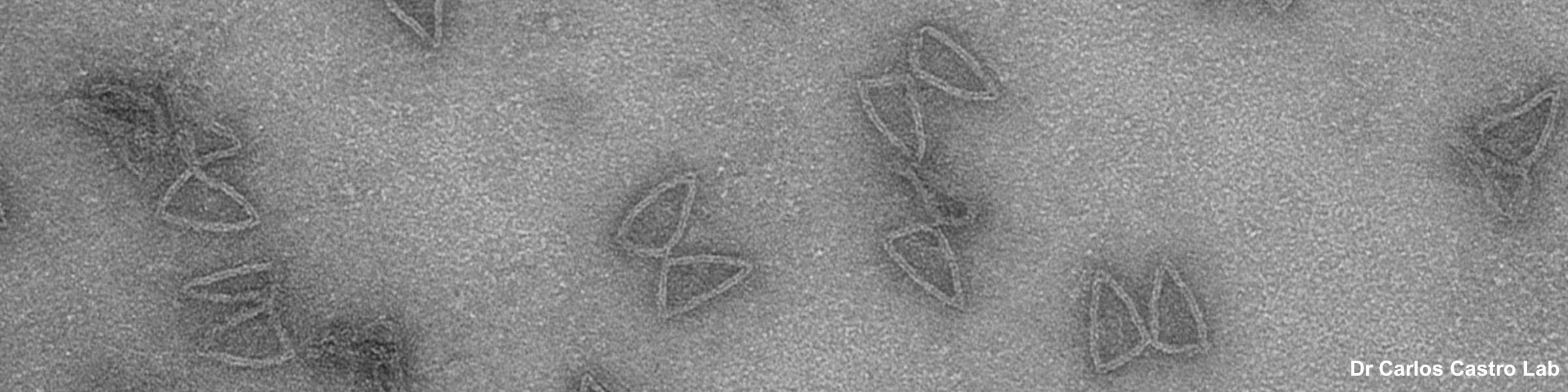
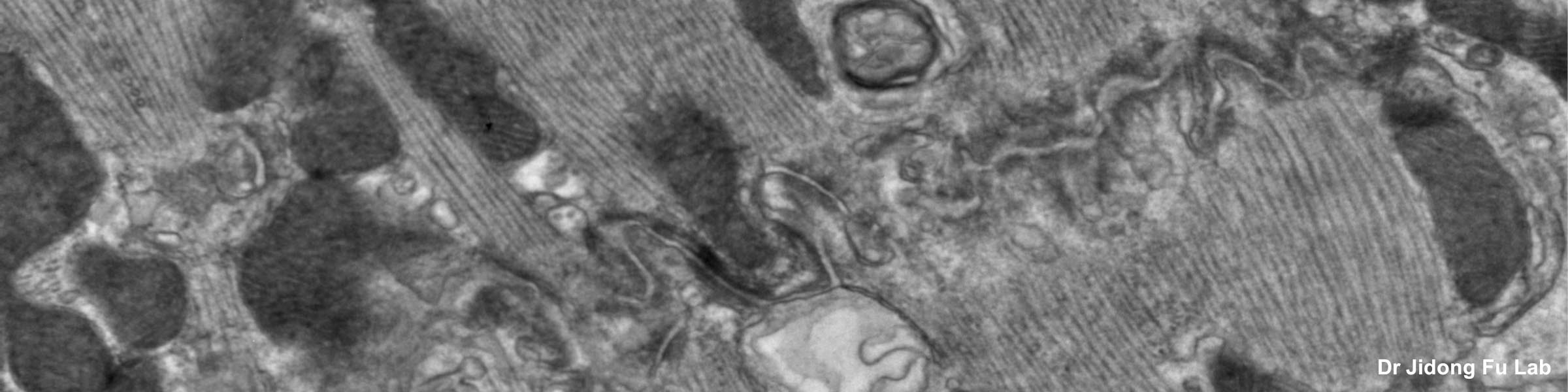
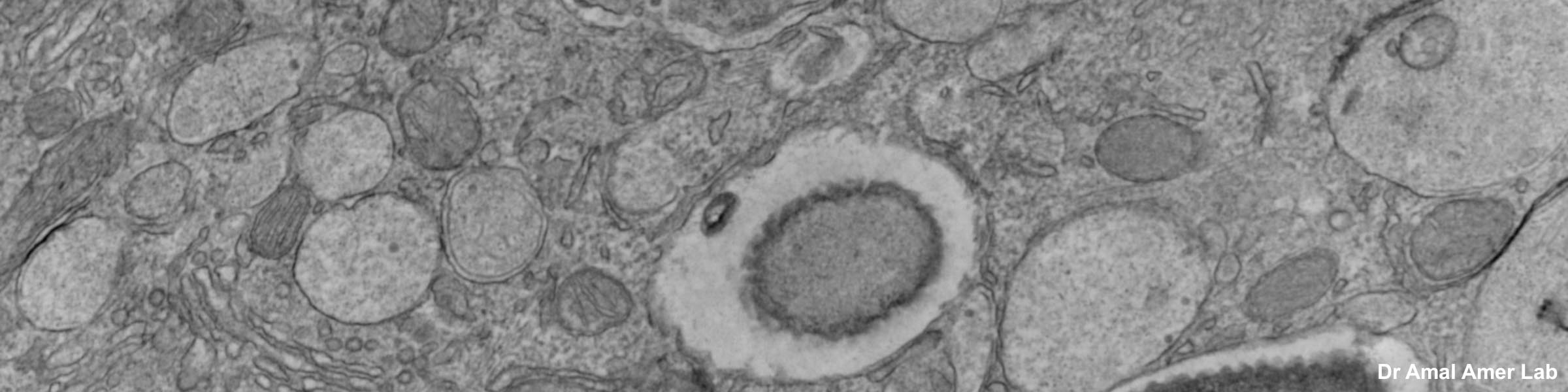
CMIF has two Confocal Microscopes for Live and Fixed Cell imaging, the Nikon A1R Confocal Microscope, and a new Nikon AXR Confocal Microscope.
Nikon AXR Confocal Microscope for Live and Fixed Cell Imaging
The Nikon AXR confocal microscope is a newly released upgrade to the A1R series. The AXR is currently located in the BSL2 area in the BRT, it is planned to be housed in the new PRC building. The AXR allows:
- Faster scanning capabilities for quicker imaging times and reduced photobleaching
- Up to 8K x 8K resolution for Galvano Scanner (2X the A1R)
- Up to 2K x 2K resolution for Resonant Scanner (2X the A1R)
- Collection of larger fields of view (FOV)
- Piezo drive capable of capturing 600 um z-stacks vs 100 um on the A1R (we are waiting the final installation of a piezoelectric stage but otherwise the system is fully functional for 4D multi color imaging)
Nikon A1R Confocal Microscope for Live Cell and Fixed Cell Imaging
ACCESS:
The Nikon A1R Live Cell Imaging Confocal Microscope is available for independent use. Before beginning your live cell imaging project, please EMAIL the CMIF staff with a detailed description of your experiment. We will be able to provide you with advice on how to prepare your samples for optimal live cell imaging results. Training is performed on an individual basis using the researchers’ samples and instrument rates apply.
BSL-2 training from EHS is required for all researchers wishing to perform live cell imaging at the CMIF. A signed form must be submitted to the facility before the start of any live cell imaging project.
RATES:
Please refer to our rates page.
INFORMATION:
The Nikon A1Rsi Resonant scanning inverted confocal system has the following features and capabilities:
- The system is equipped with four laser lines at 402nm, 487nm, 561nm, 638nm for maximum flexibility in dye choice for the investigator. Four fluorescence detectors can accommodate sequential image capture of four fluorescence markers in the same sample practically simultaneously. The system also has Differential Interference Contrast (DIC) capabilities for detection of samples in the absence of fluorescence. This approach is useful when imaging samples transiently transfected with a gene encoding a fluorescent protein. The untransfected cells are easily distinguished from those expressing the fluorescent tag. Two of the four detectors are GaAsP photomultiplier tubes that offer an increased signal to noise ratio for low light (i.e. dim signal) applications.
- The climate control chamber accommodates multiwell plates (6, 12, 24, 48, 96) in addition to glass-bottomed petri dishes and multiwell chamber slides providing the researcher with maximum flexibility when choosing a culture vessel. In addition to the standard 5% CO2 gas inlet, other gases can easily be used to induce oxidative stress/damage in cells and tissue.
- The Nikon Perfect Focus System (PFS) will automatically preserve focus using an infrared beam of light to maintain a set distance between the objective lens and the cover glass to combat thermal changes. The PFS will also correct for focus differences during multi-point (multiple stage position) observations over long periods of time when using the fully automated, motorized stage.
- The resonant scanner allows for high speed image capture at 30 full frames per second using a standard digital image format of 512x512 pixels. Faster speeds can be achieved (up to 400 frames per second) at an image format of 32x512 pixels. Shorter illumination times result in less cell damage due to light exposure (phototoxicity) in the live specimens during observation. The resonant scanner can be used in conjunction with the standard confocal galvanometric scanner to perform photoactivation experiments (i.e. proteins that fluoresce only after activation by light such as PA-GFP), or for Fluorescence Recovery After Photobleaching (FRAP) assays. FRAP assays can be used to examine protein transport malfunction in cancer cell lines. Simultaneous excitation or photoablation can be performed while imaging when using both scanners so that even the most immediate events are detected.
- The Piezo-Z stage insert is capable of axial scanning at a rate of 100 micrometers per second. This component can also be combined with use of the resonant scanner to perform 3-dimensional imaging of live cells. The piezo-Z stage can be used in conjunction the tiling capabilities of the system to provide a larger field of view in three dimensions.
- The system is equipped with a 32-channel PMT spectral detector that provides spectral imaging and linear unmixing capabilities. Accurate spectral unmixing provides maximum performance in the separation of closely overlapping fluorescence spectra and the reduction or elimination of autofluorescence. Computational algorithms and high-speed data processing enable real time or “live” unmixing during image acquisition.
- An EMCCD Hamamatsu camera is available for widefield imaging of fluorescence markers in the following ranges: blue (DAPI), green (GFP), red (mCherry) and far-red (Cy5).
- NIS-Elements C control software and fully motorized stage enable high content imaging and analysis useful for drug discovery applications.
Technical information regarding this instrument is available by following this link: Nikon Technical Information.